Is artificial blood the future of emergency medicine, or just another experimental promise?
A Cautious Look at Japan’s Artificial Blood: Possibilities, Uncertainties, and the Unknowns
In recent years, researchers in Japan have been working on a type of artificial blood known as haemoglobin vesicles (HbVs), which has caught the attention of the medical world. Touted as a universal alternative to donor blood, these tiny, lab-made particles carry oxygen without the need for blood type matching. However, while the concept feels hopeful, it’s still in its infancy, and for most of us, the tried-and-tested options remain safer bets.
What Exactly Are Haemoglobin Vesicles?
A Closer Look
HbVs are microscopic spheres made from expired donated blood and filled with purified haemoglobin, the protein that delivers oxygen in our red cells. They’re enclosed in a fat layer designed to mimic red blood cells’ function. Because they lack blood-type markers, in theory, they can be used on anyone.
However, they are not fully synthetic. They still rely on human blood. Despite careful cleaning and virus inactivation, those seeking truly blood-free treatments may find this distinction important.
Helpful Definitions
- ABO Compatibility: HbVs, stripped of red cell antigens, are meant to be compatible with any blood type.
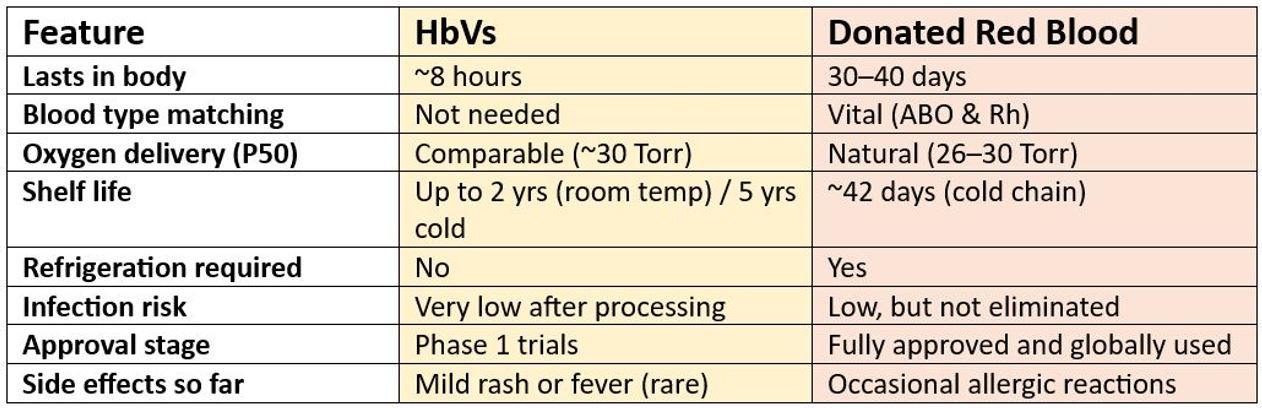
- Half-Life: HbVs work for around 8 hours, which is enough for short-term use, but far less than real red blood cells, which last 30–40 days.
- P50 Value: This shows how well haemoglobin lets oxygen go to tissues. HbVs are designed to behave like natural red blood cells (~30 Torr).
Where It Could Help — But with Caveats
HbVs are envisioned to assist in:
- Disaster zones where refrigeration and blood typing are tough.
- Military or rescue missions, where rapid, universal help is vital.
- Remote clinics without cold storage.
Yet, remember:
- It’s a stop-gap, not a complete replacement.
- We’ve only seen limited human trials — no long‑term data.
- Production could be expensive and challenging.
- HbVs can’t clot blood, support immunity, or restore volume like whole blood does.
The Development Journey
- 2022: Small safety trial (100 mL doses) in healthy volunteers, no major concerns.
- 2025: Phase 1 extended to 16 volunteers (up to 400 mL), safety still reassuring.
- 2025–26: Larger Phase 2/3 trials expected.
- 2030 or later: If all goes well, HbVs might reach hospitals, but timelines could slip.
How Do HbVs Achieve? — A Quick Comparison
Lessons from the Past
Earlier artificial blood efforts (like PolyHeme or perfluorocarbons) failed due to short lifespan, side effects or breakdown. HbVs aim to learn from those mistakes, but the proof of success is still ahead.
What We Don’t Yet Know
- How will HbVs respond in complex cases, such as surgery or chronic anaemia?
- What about repeated doses over time?
- Can manufacturers scale up production affordably?
- Will regulators in countries outside Japan approve them?
With these uncertainties, it’s wise to remain realistic.
Because Some Tools Are Proven and Already Here
While HbVs are being developed, there’s no need to wait for something untested. Multiple already‑available alternatives have strong safety records:
- Cell salvage systems: collect and return your own blood during surgery.
- Volume expanders: saline, colloids or other fluids that support circulation.
- Pre‑operative autologous donation: storing your own blood before planned surgery.
- Acute normovolaemic haemodilution: remove and later return some of your blood during surgery.
- Erythropoiesis‑stimulating drugs and iron therapy: boost red cell production over weeks.
- Tranexamic acid and other agents: help stop bleeding during and after surgery.
Institutional programmes like Johns Hopkins and Penn Medicine have shown that routine use of these methods can reduce or even avoid the need for transfusions, and often lead to better outcomes.
Final Thoughts
HbVs are intriguing, and could one day help fill gaps when supplies, refrigeration or blood matching aren’t options. But at present, they’re unproven, limited, and expensive.
For now, if you’re concerned about transfusions or want to avoid them, established alternatives offer reliable, well‑tested safety. Innovation inspires hope, but when lives are at stake, evidence is everything. Until HbVs have that, we should trust what we already know works.
Looking for safer, proven alternatives you can use today?
While artificial blood remains experimental, many established options already exist to reduce or avoid transfusions. From cell salvage to iron therapy, these methods are helping thousands of patients every year. Discover them here: Alternatives to Blood Transfusions
Source:
Just a friendly reminder that no information in this publication constitutes legal or medical advice from My Medical Choice or any of our affiliates and the contents of this document are for educational and support purposes only.